Do you have heavy eyelids that make you look tired all the time?
Renew MedSpa is now offering UPNEEQ®, an FDA-approved a prescription eyedrop used to treat low-lying lids (acquired blepharoptosis) in adults.
Product Information
UPNEEQ® is a simple and effective way to treat low-lying eyelids and is an alternative to blepharoplasty cosmetic surgery. No surgery, no down-time – just brighter, bolder, and well-rested and fresh looking eyes. It’s like a cup of coffee for your eyes!
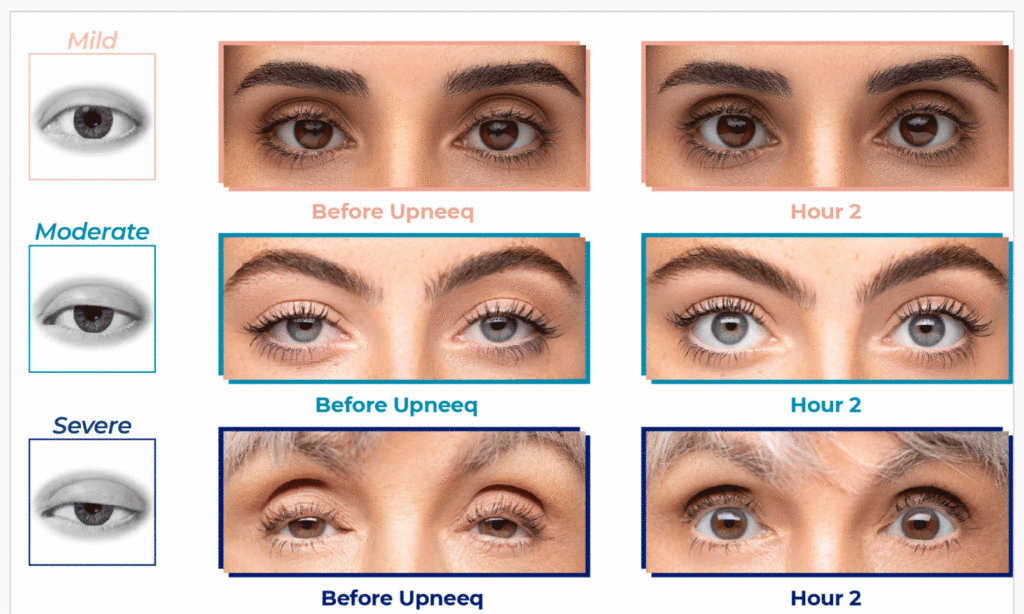
Have you noticed a change in the appearance of your eyes, where your eyelids are starting to droop? You may have acquired ptosis, or low-lying lids. Acquired ptosis (low-lying lids) is a common medical condition that:
- Affects adults of all ages but occurs more often with increased age.
- Usually occurs when the muscles in the eyelid stretch and weaken, causing the upper eyelid to droop and causing asymmetrical eyes.
- May be caused by other issues, such as cataract surgery, contact lens wear, or an underlying medical condition. It could also be a sign of a more serious medical condition.
- In clinical trials, Upneeq® helped patients with acquired ptosis see more—on the first day of treatment! 87% of patients had some form of improvement. 74% of patients had at least a 50% improvement on Day 14 (2 hours after applying Upneeq®).
UPNEEQ® (oxymetazoline hydrochloride ophthalmic solution), 0.1 % contains oxymetazoline hydrochloride, an alpha adrenoceptor agonist and is FDA approved for the treatment of acquired blepharoptosis (low-lying eyelids) in adults. Acquired ptosis can develop later in life and usually occurs when muscles in the eyelid stretch and weaken, causing the lid to droop. Eyelid ptosis may be caused by other issues, such as cataract surgery, contact lens wear, or an underlying medical condition. Eyelid ptosis can affect eyesight by not only blocking your vision, but also by reducing field of vision
Each prescription contains 45, 0.3 mL single use applicators in individual child-resistant foil pouches.
TSA Friendly: Yes
How to use
Each vial of Upneeq contains enough solution to allow for one drop in each affected eye.
- Wash hands thoroughly.
- Open the foil packet and remove the single-use UPNEEQ® vial and shake vial.
- If you wear contact lenses, remove them before applying Upneeq ® eyedrops. You may put contact lenses back in 15 minutes after applying Upneeq ®.
- Twist off the top of the vial.
- Tilt your head back and apply one drop of UPNEEQ® to each eye.
- Keep your head tilted back, roll your eyes around, and blink for 15-20 seconds. Doing this ensures the product has time to coat the eye and take effect. If more than one topical ophthalmic drug is being used, the drugs should be administered at least 15 minutes between applications.
- Discard vial.
- Enjoy up to 8 hours of bigger, bolder, and brighter eyes.
Purchase Options
- $240 – One box of UPNEEQ® (45 treatments) – Regular price $250
- $55 for 10 treatments
- $30 for 5 treatments
- $340 – One box of UPNEEQ® (45 treatments) and one Revision Skincare D·E·J Eye Cream® 0.5 fl oz
- $415 – One box of UPNEEQ® (45 treatments) and one FACTORFIVE Anti-Aging Eye/Lash Cream
- $310 – One box of UPNEEQ® (45 treatments) and a Lash Lift treatment
How do I purchase UPNEEQ®?
You can purchase UPNEEQ® at any appointment you have scheduled at Renew MedSpa, or you can stop in during open hours. It is best to call before arriving to ensure we are available to help you when you arrive. We may be working with another client and not able to greet you promptly without an appointment or heads up.
Storage requirements
Upneeq ® should be stored at 68°F-77°F (20°C-25°C) and should be protected from excessive heat. Keep out of reach of children.
Ingredients
UPNEEQ ® (oxymetazoline hydrochloride ophthalmic solution), 0.1%.
contains oxymetazoline hydrochloride, an alpha adrenoceptor agonist. UPNEEQ ® is an aseptically prepared, sterile, nonpreserved ophthalmic solution. Each mL of UPNEEQ ® (oxymetazoline hydrochloride ophthalmic solution) 0.1% contains 1 mg of oxymetazoline hydrochloride, equivalent to 0.09 mg (0.09%) of oxymetazoline free base. The ophthalmic solution contains the following inactive ingredients: calcium chloride, hydrochloric acid (used to adjust pH to 5.8 to 6.8), hypromellose, magnesium chloride, potassium chloride, sodium acetate, sodium chloride, sodium citrate, and water for injection.
FAQ
What is Upneeq®?
UPNEEQ® (oxymetazoline hydrochloride ophthalmic solution), 0.1% is a prescription eyedrop used to treat low-lying lids (acquired blepharoptosis) in adults.
How many days will my Upneeq® prescription last?
One box of Upneeq includes 45 single-use vials that will last 45 days when used daily. You may use as needed instead of daily, if desired.
What are the side effects of using Upneeq®?
The most common adverse reactions with UPNEEQ® (occurring in 1-5% of patients) are eye inflammation and/or eye redness.
Can I use Upneeq (oxymetazoline) with other eye products?
Yes, but don’t administer Upneeq (oxymetazoline) and other eye products in the same eye at the same time. Separate other eye drops from Upneeq (oxymetazoline) by at least 15 minutes. This makes sure your eyes can absorb each medication fully.
What should I do if I forget to use Upneeq for a day or two?
You can use Upneeq daily or as needed. For constant daily results, daily use is suggested. If you miss a day or two of use, for those days, you will not see the Upneeq® results.
How long does it take Upneeq® to work?
About 15 minutes, although many patients see results as early as 5 minutes.
How can I tell that I’m buying authentic Upneeq®?
Each UPNEEQ® kit is factory-sealed by the manufacturer. The back of the box is marked with a lot number and expiration date that matches the information on the bottle.
What warnings and precautions are associated with Upneeq®?
- UPNEEQ® is a type of medication that may affect your blood pressure. If you have heart disease, uncontrolled high or low blood pressure, or feel faint at rest or when quickly standing up, you should call your medical provider if your symptoms get worse.
- Patients with reduced blood flow to the brain or heart, or patients who experience eye or mouth dryness due to an immune system disorder (Sjögren’s syndrome), should use care when taking UPNEEQ®. Call your medical provider immediately if you feel your symptoms may be getting worse.
- UPNEEQ® may increase the risk of eye pressure due to fluid buildup (angle-closure glaucoma) in patients with untreated narrow-angle glaucoma. Call your medical provider immediately if you feel increased pressure in your eye after using UPNEEQ®.
- Do not let the tip of the UPNEEQ® vial touch your eye or any other surface. This can help prevent eye injury or contamination. Each UPNEEQ® vial is for one-time use and should be discarded after being used.
What should my medical provider know before prescribing me Upneeq®?
- UPNEEQ® belongs to a class of medication (alpha-adrenergic agonists) that may affect your blood pressure. Use UPNEEQ® carefully if you currently take an alpha-adrenergic antagonist medication to treat heart disease or an enlarged prostate. Patients taking beta-blockers, or other medications to treat hypertension or an abnormal heartbeat, should also be careful when using UPNEEQ®.
- Patients who use a certain class of antidepressant medication (monoamine oxidase inhibitors) should also be careful when using UPNEEQ®, as it may affect the way your body absorbs the medication
Can I return Upneeq® if I change my mind?
Federal regulations forbid accepting returns of prescription products.
What temperature can Upneeq® withstand to remain stable?
UPNEEQ® should be stored at 68°F – 77°F (20°C – 25°C) and should be protected from excessive heat.
Please contact us in one of the following ways to schedule an appointment.
- Book online
- Phone: 952-212-1268
- Text: 952-522-5868
- Request appointment
- View our links

